Strategically Focused Research Network (SFRN) on Biologic Pathways of Chronic Psychosocial Stressors on Cardiovascular Health
Key Dates
| RFA Posted: | September 23, 2022 |
| Required Pre-proposal Deadline: | November 17, 2022 |
| Application Deadline: | January 19, 2023 |
| AHA 2-Phase Peer Review: | February and March 2023 |
| Notification of Awards: | March 2023 |
| Award Start Date:: | April 1, 2023 |
Related Webinars - Register today!
Proposal Central webinar - December 8, 2022, 12-1 p.m. CST
Q&A only webinar - January 11, 2023 12-1 p.m. CST
Applicant Requirements
As a reminder, any individual who is applying as a Center Director or a Project PI must be an AHA Member. Join or renew when preparing an application in Proposal Central, online, or by phone at 1-888-242-2453 or 972-349-5803. Membership processing takes three to five days; do not wait until the application deadline to renew or join.
Required Pre-Proposal
Each Center director must submit a pre-proposal by November 17, 2022 that provides:
- Name and institution of the Center director and each Project Investigator (PI)
- Network title; and title and performance site of each proposed project
- If required, the mechanism through which the application plans to meet partnering requirements (see the Additional Expectations and Opportunities and Institutional Eligibility/Location of Work sections)
As part of the required pre-proposal, if the submitting institution or a partnering institution is not a research-intensive institution of higher learning, the lead for that institution, must upload a letter from a senior institutional official (for example, a president, provost, dean, etc.) indicating that the institution meets the definition of a non-research-intensive institution as stated in the "Additional Expectations and Opportunities" section. The AHA will review for compliance, and will not permit a non-complying institution to submit a full application.
This administrative review is part of the pre-proposal process, which is required and can prevent an applicant from moving forward. Even though the pre-proposal is required, each Center and Project applicant should begin planning and designing their applications before the pre-proposal deadline to maximize the amount of time available to develop their full application.
Purpose
The AHA announces a request for applications (RFA) for the Strategically Focused Research Network (SFRN) on Biologic Pathways of Chronic Psychosocial Stressors on Cardiovascular Health.
Cardiovascular disease (CVD) is the leading cause of death in the United States, accounting for over 870,000 deaths in the United States in 2019.1 An extensive body of evidence generated over several decades has demonstrated that a large number of behaviors (smoking, insufficient physical activity, diet, etc.) and risk factors (blood pressure, cholesterol levels, diabetes, etc.) play important roles in the development of cardiovascular disease and mortality.1 In more recent years, an appreciation of the influence of various forms of stress on cardiovascular health has also emerged. Stress can take many forms, and both acute and chronic stress can result in poor health outcomes. Stressors may act either independently or synergistically with each other to enhance the severity of cardiovascular risk factors and outcomes. With regard to CVD, chronic stress is recognized as an independent risk factor for its development and increases morbidity and mortality for those with existing coronary artery disease.2
Psychosocial stress (PSS) is known to have adverse effects on cardiovascular health. Psychosocial stressors are those having both a psychological and social component, and include conditions and situations such as work, relationship, or marriage difficulties; living in isolation; a lack of social support or basic resources; major life events; being subjected to discrimination and systemic racism; and other conditions. INTERHEART was a seminal, global study demonstrating an association of psychosocial risk factors with cardiovascular health, namely the risk of acute myocardial infarction.3 This study was unique in that it assessed these factors in individuals from 52 countries; it demonstrated a strong correlation for multiple psychosocial stressors and myocardial infarction (MI) in comparison to the age-matched control group. In addition, the association of PSS and MI was consistent across regions and ethnic groups, and was similar in men and women.
In considering specific psychosocial stressors, a large body of evidence demonstrates an association between cardiovascular health and work stress.4 Early reports of job-related stress and CVD found that men who experienced low reward at work compared to the effort they expended were more than twice as likely to experience coronary heart disease.5 Around the same time, a study of Swedish working men found that those who had had an initial myocardial infarction were significantly more likely to have experienced job stress.6 Many subsequent studies have demonstrated the association between job stress and cardiovascular health.4,7
Caregiving is another prominent psychosocial stressor, and caregivers have a greater risk of developing CVD than non-caregivers.8 Specifically, caregivers of people with dementia experience high levels of stress and depressive symptoms.9,10 Likewise, stress associated with caring for a sick spouse nearly doubles the risk of CVD mortality.8
Chronic psychiatric conditions have also been tied to CVD. Meta-analyses have found depression to be independently associated with an increased risk of coronary heart disease and MI.11,12 Similarly, anxiety and post-traumatic stress disorder increase the incidence of CVD, and the increase in risk is comparable to that seen in behavioral risk factors such as smoking and obesity.13,14
Evidence has likewise developed indicating that PSS associated with discrimination and racism lead to an increase in cardiovascular risk factors and worsened cardiovascular outcomes.15 This is especially pronounced for African Americans who have a higher risk of hypertension than other racial groups.16 For example, the Jackson Heart Study found a relationship between lifetime discrimination and incident hypertension over the course of this longitudinal study.16 These psychosocial stressors likely contribute to elevated cardiovascular morbidity and mortality in Black men and women.16 Other work has explored the relationship between cumulative PSS and cardiovascular health, and shown differences with race and ethnicity.17
Initial physiologic events related to psychosocial and other stressors are generally understood. Stress activates the sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal (HPA) axis and a number of subsequent physiologic responses. These include neutrally-mediated increases in vasoconstriction, heart rate and cardiac output; the release of systemic catecholamines facilitates this effect. With persistent activation such as that seen with chronic PSS, dysregulation and adverse effects occur. For instance, in addition to cardiac dysregulation via sympathetic activation, a broad array of immune response pathways are activated, leading to a systemic inflammatory response; this increased inflammation has been identified as a link between stress and atherosclerosis.18-21 More recent work on the connection between stress and CVD has explored how the brain responds to one’s environment. As examples, the metabolic activity of the amygdala may be predictive of CVD through a pathway of increased bone marrow activity and arterial inflammation,22 while decreases in brain-derived neurotrophic factors appear to relate to myocardium dysfunction and behavioral anomalies in a mouse model of PSS.23 The impact of stress on the various systems of the body (for example, the HPA axis, nervous system, immune dysregulation) suggests multiple opportunities for targeted interventions and therapeutic strategies.
Neuroendocrine markers such as cortisol, epinephrine, norepinephrine and dehydroepiandrosterone sulfate correlate with PSS.24,25 Similarly, inflammatory markers such as C-reactive protein, tumor necrosis factor a (TNFa), interleukin-6 and markers of oxidative stress have been shown in a number of studies to be elevated during chronic stress.21,26 Whereas animal models have also been critical in identifying the particular pathways activated in these conditions and their effects on cardiac and vascular function,26-28 key questions remain about signaling specificity in response to varying psychosocial and other stressors, potential unique determinants with regard to the impacted organ or function, and the relevant time course(s) for the key signaling molecules and pathways.
While our understanding of the impact of PSS on cardiovascular health has increased in recent years, large gaps in our fundamental understanding of PSS and CVD remain. A strong need exists for further elucidation of the cellular and molecular signaling pathways activated in response to chronic psychosocial stressors, and research about stress interventions at population levels and how interventions relate to underlying molecular mechanisms.
Network Overview and Structure
General overview:This SFRN on the Biologic Pathways of Chronic Psychosocial Stressors on Cardiovascular Health will consist of at least three centers - each of which will propose novel research studies to address this issue. The AHA expects funded centers to collaborate on solving the core issues underlying this problem, including the development of a common network-wide collaborative project (see below).
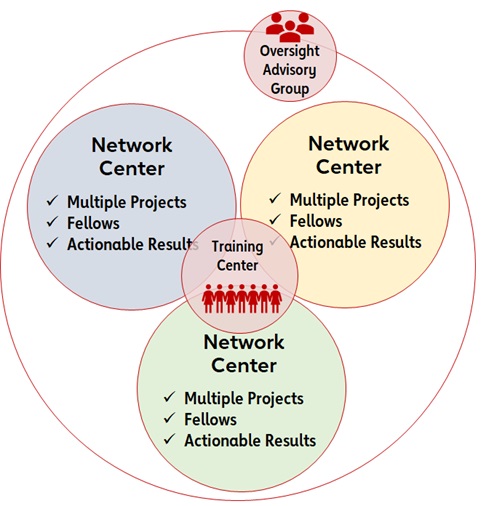 Network centers: Each center application will include three research projects from at least two science disciplines (basic, clinical, population health research). Given the critical need for a fundamental understanding of the mechanisms that drive the cardiovascular effects of chronic PSS, two of the proposed projects must be basic science-focused. Proposed projects should be complementary in addressing the mechanistic aspects of PSS and cardiovascular health. Projects may all be from a single institution, or they may be from multiple institutions. Each research project will be led by a project PI and must have the necessary research team, required infrastructure and ability to conduct the stated research. One overall Center director will also be a key component of each network center. Each Center’s director will facilitate activities within their center and work closely with the other network center directors to facilitate activities across the Network, including end-of-network deliverables.
Network centers: Each center application will include three research projects from at least two science disciplines (basic, clinical, population health research). Given the critical need for a fundamental understanding of the mechanisms that drive the cardiovascular effects of chronic PSS, two of the proposed projects must be basic science-focused. Proposed projects should be complementary in addressing the mechanistic aspects of PSS and cardiovascular health. Projects may all be from a single institution, or they may be from multiple institutions. Each research project will be led by a project PI and must have the necessary research team, required infrastructure and ability to conduct the stated research. One overall Center director will also be a key component of each network center. Each Center’s director will facilitate activities within their center and work closely with the other network center directors to facilitate activities across the Network, including end-of-network deliverables.
Oversight Advisory Committee: An Oversight Advisory Committee (OAC) will facilitate the success of this SFRN. The OAC will comprise volunteers who are subject matter experts in the focus areas.
Representative Approach to Responding to this RFA
The intent of this initiative is to support a collaborative network of researchers whose collective efforts will lead to a greatly enhanced understanding of the mechanisms underlying the impact of chronic PSS on cardiovascular health, such as actions related to vascular dysfunction, arrhythmias, and myocardial dysfunction, among others. Although here are some potential areas of investigation, the list below is not exhaustive and is not meant to direct applicants to a particular area of study:
- The identification and assessment of cellular and molecular mediators, including changes in magnitude and time course of signaling events, and extent to which they may be unique depending on the psychosocial stressor.
- The identification of the extent to which distinct signaling pathways may predominately associate with particular cardiovascular consequences of PSS.
- The identification of novel biomarkers that correlate with specific psychosocial stressors and/or specific cardiovascular changes.
- Clinical interventions designed to test a mechanistic hypothesis related to PSS and/or its mitigation through the proposed intervention.
- Studies to understand the mechanisms through which stress management can be used as a primary prevention strategy for the general population.
- Research on social and environmental factors that cause stress and how those then impact cardiovascular health.
- Research on the nuance and complexity of discrimination and how it affects cardiovascular health.
Study Population(s)
- For studies involving human subjects, projects must include study participants who are underrepresented and/or underserved with regard to healthcare delivery. The proportion of these individuals in proposed studies should be reflective, at minimum, of their representation in the local/regional population from which subjects will be recruited.
- It will be important for applicants to design studies that incorporate both realistic recruitment goals and sufficient statistical power to ensure valid results.
Additional Expectations and Opportunities
- In keeping with the AHA’s commitment to supporting diverse researchers and institutions, applicants must meet one of the following conditions. A letter is required as part of the required pre-proposal confirming that the institution meets these conditions:
- Center applications must originate from investigators at academic institutions that primarily serve individuals from groups who are under-represented in science (for example, historically black colleges and universities (HBCUs), Hispanic-serving institutions (HSIs) or tribal colleges and universities or investigators at a non-research-intensive institution as defined by the National Institutes of Health (NIH) (an average of less than $7.5 million in total NIH funding over the past three fiscal years).
Or - For center applications originating from research-intensive institutions, those institutions must partner with an institution from one of the two categories noted in the preceding paragraph. Investigators from these partnering institutions must be included in a substantive manner (see the Projects section).
- Center applications must originate from investigators at academic institutions that primarily serve individuals from groups who are under-represented in science (for example, historically black colleges and universities (HBCUs), Hispanic-serving institutions (HSIs) or tribal colleges and universities or investigators at a non-research-intensive institution as defined by the National Institutes of Health (NIH) (an average of less than $7.5 million in total NIH funding over the past three fiscal years).
Application Details
Network Center Application Details
Duration: Four years
Number of awards:The AHA anticipates awarding at least three network center grants to establish this SFRN. Awardees will be selected based on scientific merit and how each group aligns with the AHA’s mission and goals.
Collaborative project: During year 1 of the network, the Centers will be required to develop a network-wide collaborative project, with cooperation from the network OAC. The collaborative project will start in year 2. The AHA has set aside money for this effort not to exceed $1.2 million for the network. The AHA will make more details about the collaborative project available after the Centers are named.
Award amount: The maximum budget amount that a Center applicant may request is $4,433,333. The AHA reserves the right to determine the final award amount for competitive projects based on need and potential impact.
Appropriate budget items:
- Salary and fringe benefits for the Center director, PIs, three named fellows, collaborating investigator(s), and other participating research staff or faculty.
- Project-related expenses such as salaries for technical personnel essential to the conduction of the project, as well as supplies, equipment, travel and publication costs in accordance with institutional and AHA policies.
- Centers should use award dollars to pay for traveling to two required face-to-face (as feasible) network-wide meetings each year as well as and other meetings where SFRN research is presented. The AHA will convey additional details on bi-annual meetings to awarded centers following award activation. Centers should anticipate hosting at least one of the meetings on a rotating basis. The purpose of both meetings is to share results across the network and identify and act on potential collaborative opportunities. There will be virtual meetings if face-to-face travel is not available. The AHA will provide more information will be provided upon award and once travel options become clear.
- If allowed by the program, institutional indirect costs for operating expenses may be charged up to 10% of the total expenditures each year on awards at the awardee institution. Any subcontract awardee institution (if applicable) is allowed institutional indirect costs up to 10% of the total expenditures of the subcontract. The awardee institution may not charge indirect costs on the direct costs of a subcontract.
| Sample Center Budget | Central Totals |
| Projects Three projects from at least two science disciplines for four years with amaximum of $3.32 million to be divided among three projects. It is not a requirement to spend funds equally across projects or years. |
$3.32 million |
| Fellows Each center must train three postdoctoral fellows over the four-year grant period (for example, one fellow in years 1 and 2, one fellow in years 2 and 3, and one fellow in years 3 and 4. Up to $65,000 per fellow per year: salary + health insurance/fringe. Fellows must maintain a minimum of 75% effort to research training. See additional requirements for fellow appointment below. |
$390,000 |
| Center leadership A maximum $50,000 salary plus fringe/benefits per year to cover efforts associated with directing the Center. One center director must commit at least 20% effort for these efforts. |
$250,000 |
| Center travel costs Covers travel for Center personnel to attend network meetings and other integration activities. $10,000 per year must be allocated to center travel. |
$40,000 |
| One-time hosting of a face-to-face scientific meeting | $30,000 |
| Direct costs (total) Research dollars |
$4.03 million |
| Indirect costs AHA policy allows for a maximum of 10% for indirect costs. |
$403,000 |
| Total | $4.433 million |
Note for center applicants: There may be only one center director at each Center. This person will be responsible for the progress of the projects and overseeing the total budget for their grant. If awarded, the PI and the institution assume an obligation to expend grant funds for the research purposes set forth in the application and in accordance with all regulations and policies governing the grant programs of the AHA.
Directors and PIs of projects of the Centers:
- Must possess an MD, PhD, DO, DVM or equivalent doctoral degree at the time of application.
- Must have a faculty or staff appointment.
- May hold another AHA award simultaneously.
- Must demonstrate a 20% minimum effort requirement for the director and a 10% minimum effort requirement for the PI of center projects. These responsibilities are mutually exclusive; i.e., if a center director also serves as a Project PI, they must contribute a combined effort of 30%. Each named director and PI must be able to commit the minimum effort required and may not split these efforts across more than one person.
Directors must have one of these designations:
- U.S. citizen.
- Permanent resident.
- Pending permanent resident (must have applied for permanent residency; filed Form I-485 with U.S. Citizenship and Immigration Services and have received authorization to legally remain in the U.S., having filed an Application for Employment Form I-765).
- G-4 visa – family member of an employee of international organizations and NATO.
PIs of proposed projects must have one of these designations:
- U.S. citizen.
- Permanent resident.
- Pending permanent resident (must have applied for permanent residency; filed Form I-485 with the U.S. Citizenship and Immigration Services; and have received authorization to legally remain in the U.S., having filed an Application for Employment Form I-765).
- E-3 visa – specialty occupation worker.
- H1-B visa – temporary worker in a specialty occupation.
- O-1 visa – temporary worker with extraordinary abilities in the sciences.
- TN visa – NAFTA professional.
- G-4 visa - family member of an employee of international organizations and NATO.
Named Fellows
The AHA’s aim is to help end historical structures and workplace cultures that advertently or inadvertently treat people inequitably based on race, ethnicity, gender, sexual orientation, age, ability, veteran status or other factors. Therefore, at least 50% of the fellows named must be from a racial or ethnic group that is under-represented in science (Black/African American; Hispanic/Latino; Native American or Alaska Native; and/or Hawaiian or other Pacific Islander) or an LGBTQ+ person or a woman.
Each fellow must have one of the following designations:
- U.S. citizen.
- Permanent resident.
- Pending permanent resident (must have applied for permanent residency; filed Form I-485 with the U.S. Citizenship and Immigration Services; and have received authorization to legally remain in the U.S., having filed an Application for Employment Form I-765).
- E-3 visa – specialty occupation worker.
- H1-B visa – temporary worker in a specialty occupation.
- O-1 visa – temporary worker with extraordinary abilities in the sciences.
- TN visa – NAFTA professional.
- J-1 visa – exchange visitor.
- F-1 visa – student.
- G-4 visa – family member of employee of international organizations and NATO.
*All awardees must meet the citizenship criteria throughout the duration of the award.
A named fellow may not hold another comparable fellowship award, although the institution may provide supplemental funding. Fellows may not hold a faculty or staff appointment, with the exception of MD or MD/PhD trainees who also maintain clinical responsibilities. These fellows may hold the title of instructor or similar due to their patient care responsibilities but must devote at least 75% effort to research training.
Peer Review
General: Peer Review will be a two-phase process. Projects/Science from the Network Centers will be scored during Phase 1. Network Center applications that advance past Phase 1 will undergo a separate Phase 2 review. This review will focus on the overall vision of the center, synergy and collaborative possibilities within a Center (via the Center application) and across Centers, and the training plan and environment. Phase 2 will occur 2-4 weeks after Phase 1 review. Criteria for both phases of review follow.
Peer Review Criteria for Project Applications
Phase 1 Review
Each Project within a Center application will be scored individually according to the criteria below.
Projects: Potential impact of the project on research in the field of the designated research topic; strengths of applicant investigators (qualifications, expertise and productivity); potential for collaboration or synergy of projects; scientific content; background; preliminary studies; detailed specific aims; approach detail; analytical plan; sample size; data management; significance; innovation; individual project scientific merit; and total project coordination (within and among projects). Projects will be rated on these areas:
- Approach: Are the conceptual framework, design, methods and analyses adequately developed, well-integrated, well-reasoned and feasible (as determined by preliminary data) while also appropriate to the aims of the project? Does the applicant acknowledge potential problem areas and consider alternative tactics? Does each applicant develop a plan for the interoperability of data between Centers and with national or international standards?
Note: Applicants must explain how relevant biological variables, such as sex, are factored into the research design, analysis and reporting. Furthermore, very strong justification from the scientific literature must be provided for applications proposing to study only one sex. - Innovation: Is the project original and innovative? For example: does the project challenge existing paradigms and address an innovative hypothesis or critical barrier to progress in the field? Does the project develop or employ novel concepts, approaches, methodologies, tools or technologies for this area?
- Investigator(s): Are the investigator(s) appropriately trained and well-suited to carry out this work? Is the work proposed appropriate to the experience level of the PI and other researchers? Does the investigative team bring complementary and integrated expertise to the project (if applicable)? Project PIs must dedicate at least 10% effort to the project.
- Significance: Does this study address an important problem related to PSS and CVD? If the aims of the application are achieved, how will mechanistic understanding of mediators related to PSS be advanced? What will be the effect of these studies on the concepts, methods and technologies that drive this field?
- Environment: Does the scientific environment in which the work will occur contribute to the probability of success? Do the proposed studies benefit from unique features of the scientific environment, or subject populations, or employ useful collaborative arrangements? Is there evidence of institutional support?
- Impact: How does the project relate to and support the mission of the AHA to be a relentless force for a world of longer, healthier lives?
- Synergy: How does this project enhance the Center and additional science project(s)? Does this project enhance the likelihood that the collective Center outcomes will exceed outcomes of the individual sum of its distinct components? For more information, please see the Center Science Vision and Synergy page. Only projects that demonstrate synergy will move forward to phase 2.
- Lay summary/summary for non-scientists: How well written is the lay summary in explaining to a non-scientist audience the research proposed and its importance? Does the lay summary adequately explain the major health problem being addressed by this study? Does it provide specific questions and how the projects will address them? Does it provide information on the overall impact of this work and the potential advances in the field? Does it relay how the proposal supports the mission of the AHA?
Peer Review Criteria for Center Applications
Phase 2 Review
Each Network Center moving beyond phase I review will be scored on:
- Synergy: A clear vision of scientific direction. A Center should be viewed as a group of interrelated research projects, each of which is not only individually scientifically meritorious, but also complements the other projects and contributes to an integrating theme. Describe the rationale for the total program. Explain the strategy of achieving the objectives of the overall program and how each individual project relates to the strategy. Describe the synergies and interactions among projects and their investigators. Is there evidence of synergy among the projects and training component of the Center?
- Collaboration: Is there a history of collaboration, as well an ability and commitment to collaborate with other institutions, investigators and within the applicant institution as well as within the awarded Network? Is there a defined and detailed process for collaboration with other sites in addition to within and among the proposed projects, along with plans to actively participate in a collaborative network? Is there evidence of formal training in leadership skills with an emphasis on collaborative leadership? What collaborations do you envision between investigators working on individual projects?
- Interaction plan within and among this Network and other AHA Networks (if appropriate): Is there a plan for and commitment to sharing knowledge and methods, providing a stimulating atmosphere for research collaborations, and providing networking opportunities for trainees? Are there cited strategies for communication and interaction among the Centers? Centers proposing clinical projects must document that they have a sufficient volume of patients from all identified study populations to achieve robust results.
- Training component: Is there a detailed plan for developing and implementing a postdoctoral training program that includes clinical (MD, DO, PharmD) or PhD training in research in the field outlined by the RFA? What are the qualifications and characteristics of current and anticipated trainees? Are there didactic and practicum training opportunities? What is the plan for the selection of prospective fellows? Will funded fellows' ongoing progress be guided via an individual development plan and evaluated at least annually? Does a plan exist for involving fellows in annual Center meetings and Center-to-Center visits, along with identifying opportunities for fellows to work with established investigators at other network Centers? How will trainees be tracked? Will there be opportunities for trainees to attend conferences and participate in meetings? Is there a ready supply of fellows documented and a history of successful fellowship training for researchers in the appropriate research topic?
- Center director: What are the qualifications of the director to provide scientific and administrative leadership for the Center? Does the director have a demonstrated ability to lead others, along with experience and commitment to the success of the Center, the projects contained within, and the network? Is there documented evidence of a willingness to collaborate with others outside their institution to share ideas, science, etc., to advance research within the intended area.
- Investigator team: What are the qualifications of each PI to provide scientific and administrative leadership for their respective projects, their demonstrated commitment, and experience in the area(s) of studies proposed? What are the qualifications of investigators, co-investigators and the research team? What kind of training experience does the investigator team have?
- Diversity of the research team: In keeping with its core values of diversity and inclusivity, the AHA is committed to broadening the diversity of investigators supported by the programmatic, multi-investigator initiatives that it offers. As such, at least 25% of key personnel of the research team must be from groups who are under-represented in science and medicine. Applicants must be able to document the diverse composition of the proposed research team, and should comment on what steps their institution(s) have taken/are taking to expand and support diverse investigators.
- Environment: What are the institutional commitments, resources, and facilities to sustain the Center? What institutional resources are available to complete the project? What analytical resources are available to the project? Is there a letter from the center director’s department head assuring the department and institution’s support of the Center along with confirmation that the center director will devote at least 20% effort toward the Center? Other Center personnel may be appointed to assist the director in the administration of the Center. However, the director will be required to devote 20% effort to the Center.
For more information on peer review of submitted applications, including information on reverse site visits, see the Peer Review section of the SFRN General Information page on the AHA SFRN website.
Applicants are prohibited from contacting AHA peer reviewers. This is a form of scientific misconduct and will result in removal of the application from funding consideration and institutional notification of misconduct.
Award selection: Final funding decisions are subject to approval by the AHA.
Relevant Policies and Requirements
Institutional eligibility/location of work: AHA awards are limited to U.S.-based non-profit institutions, including medical, osteopathic and dental schools, veterinary schools, schools of public health, pharmacy schools, nursing schools, universities and colleges, public and voluntary hospitals and others that can demonstrate the ability to conduct the proposed research. Applications will not be accepted for work with funding to be administered through any federal institution or work to be performed by a federal employee, except for Veterans Affairs employees.
The Centers are not transferable to other institutions. An institution may submit only one Center (and related Projects) application in response to this RFA. Individuals at the applicant institution who are not participating in their institution’s center and project(s) application may participate in a separate institution’s center application. Individuals other than the center director, who are participating in their institution’s center application, may participate in a separate institution’s center application. The application may include individuals and/or projects at more than one institution, provided that there is evidence supporting the likelihood of a successful interaction among research and training personnel.
It is the responsibility of the submitting institution to ensure that only one proposal is submitted for the institution, or to coordinate across several institutions to create a single application. The center director’s institution will maintain fiscal responsibility for the entire award.
Use of the AHA’s Precision Medicine Platform: The AHA encourages applicants to make use of the Precision Medicine Platform (PMP), powered by Amazon Web Services.
- The PMP supports cloud computing in a secure and private workspace and enables investigators to collaborate and analyze data securely. Each Project will receive Amazon Web Services computational credits to offset the cost of using the platform. Because the credits do not cover all costs associated with use of the PMP, funded centers will incur a monthly usage charge that they will need to factor into the award budget. The approximate monthly charge will be $100 for each month of platform use.
- Data analysis is enabled in secure workspaces by a web user interface that allows researchers to code in various languages, including R and Python, and use to statistical software including but not limited to SAS and RStudio. The most up-to-date machine learning and artificial intelligence software available from Amazon Web Services is also included. For a full list of the analytical tools available, see precision.heart.org/workspace/about. Researchers are also able to upload their own tools.
- To learn more about the PMP and how it can enable your research, please access the following videos. The first (Introducing AHA's Precision Medicine Platform), provides a high-level overview, while the second (PM Platform How-To Video), provides more detail about accessing data and analytical tools, data storage, and sharing data.
- The PMP is HIPAA and FedRAMP compliant.
Interim assessment: Awardees must report progress on a minimum annual (once per year) basis. Progress may take the form of a required written report in addition to video conferencing, phone calls, and/or face to face visits. Reporting will be focused on the achievement of stated milestones as indicated in the project timeline. The OAC reserves the right to request additional updates, site visits, or reporting.
Links and References to Relevant AHA Policies
- Public access: The AHA’s public access policy requires that all journal articles resulting from AHA funding be made freely available in PubMed Central (PMC) and attributed to a specific AHA award within 12 months of publication. It is the responsibility of the awardee to ensure the deposit of journal articles into PMC.
- Open data: Any factual data needed for independent verification of research results must be made freely and publicly available in an AHA-approved repository within 12 months of the end of the funding period (and any no-cost extension). For more information on these policies, see the AHA's Open Science Policy webpage.
- Preregistration:The AHA requires preregistration for any funded clinical trials and encourages preregistration for any studies that make an inferential claim from a sampled group or population, as well as studies that are reporting and testing hypotheses. After a project is completed, protocols and preregistration analysis plans can be used in conjunction with the final study and analysis by researchers seeking to replicate, reproduce, and build upon findings. See the AHA’s preregistration information.
- Other: The projects described can have no scientific or budgetary overlap with other funded work. Any inventions, intellectual property, and patents resulting from this funding are governed by the AHA Intellectual Property Policy for Research Funding except to the extent modified by specific intellectual property terms for this award mechanism, including financial terms, that will be communicated to awardees following the review process. The applicant/awardee and institution are responsible for compliance with all AHA research award policies and guidelines for the duration of any awards they may receive. See the Research Programs Awards Policies page for more information on this topic: AHA Policies Governing All Research Awards.
Application Submission
You must submit applications using ProposalCentral, the AHA’s online submission portal.
For explicit application instructions, see the AHA SFRN General Application Information page.
Other features of this AHA research opportunity:
- AHA awards are open to an array of academic and health professionals. This includes but is not limited to all academic disciplines (biology, chemistry, mathematics, technology, physics, etc.) and all health-related professions (physicians, nurses, nurse practitioners, pharmacists, dentists, physical and occupational therapists, statisticians, nutritionists, etc.).
- The AHA strongly encourages applications by women, people in ethnic and racial groups underrepresented in science, and those who have experienced varied and non-traditional career trajectories.
References Cited
- Tsao, et al., Circulation, 145: e153-e639, 2022
- Yao, et al., Journal of International Medical Research, 47: 1429-1440, 2019
- Rosengren et al., Lancet, 364: 953-962, 2004
- Sara, et al., J Am Heart Assoc, 7: e008073, 2018
- Bosma et al., Am J Public Health, 88: 68-74, 1998
- Theorell et al., Am J Public Health, 88: 382-388, 1998
- Slopen, et al, Plos ONE, 7(7): e40512, 2012
- Lee, et al., Am J Prev Med, 24: 113-119, 2003
- Ory, et al., The Gerontologist, 39; 177-186, 1999
- Kim & Schulz, J Aging Health, 20: 483-503, 2008
- Nicholson, et al., Eur Heart J, 27: 2763-2774, 2006
- Gan, et al., BMC Psychiatry, 14: 371, 2014
- Batelaan, et al., Br J Psychiatry, 208: 223-231, 2016
- Levine, et al., Circulation, 143: e763-e783, 2021
- Churchwell, et al. Circulation, 142: e454-e468, 2020
- Forde, et al., Hypertension, 76: 715-723, 2020
- Burroughs Pena, et al., Circulation, 139:2012–2021, 2019
- Layte, et al., Sci Rep, 9: 796, 2019
- Dar, et al., Curr Treat Options Cardiovasc Med, 21: 23, 2020
- Singh, et al., Circ Cardiovasc Imaging, 9: e004195, 2016
- Ridker, et al., N Engl J Med, 377: 1119-1131, 2017
- Hinterdobler et al, Antiox Redox Signaling 35: 1531-1550, 2021
- Tawakol, et al., Lancet, 389: 834-845, 2017
- Agrimi et al., EBioMedicine 47: 384-401, 2019
- Bellingrath et al., Stress 12: 37-48, 2009
- Levy et al., Plos One 16: e0261746, 2021
- Golbidi et al., Am J Physiol Heart Circ Physiol 308: H1476-H1498, 2015
- Costoli et al., Am J Physiol Heart Circ Physiol 286: H2133-H2140, 2004